An atom is a minimal integral particle of matter. At its center is a core, around which, like planets around the sun, electrons revolve. Oddly enough, but this smallest particle was discovered and formulated the concept of it yet
ancient Greek and ancient Indian scholars who do not have the proper equipment or theoretical base. Their calculations for many centuries existed on the basis of hypotheses, and only in the 17th century, chemical scientists could experimentally prove the validity of ancient theories. But science is rapidly moving forward, and at the beginning of the last century, physicists discovered subatomic components and particle structures. It was then that such a definition of the atom as "indivisible" was refuted. Nevertheless, the concept has already entered into scientific usage and has been preserved.
Ancient scientists believed that the atom is the smallest pieces of any matter. The physical properties of a substance depend on their shape, massiveness, color, and other parameters. For example, Democritus believed that the atoms of fire are extremely sharp, because it burns, particles of solids have rough surfaces that tightly attach to each other, water atoms are smooth and slippery, since they give fluidity fluidity.
Democritus even considered the soul of man to be composed of temporarily connected atoms, which decay when the individual dies.
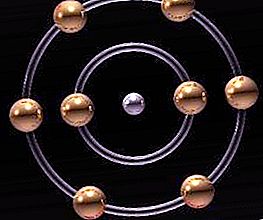
A more modern structure was proposed at the beginning of the 20th century by the Japanese physicist Nagaoka. He presented a theoretical development, which consists in the fact that the atom is a planetary system on a microscopic scale, and its structure is similar to the system of Saturn. Such a structure turned out to be erroneous. The Bohr-Rutherfried atom model turned out to be closer to reality, but even she failed to explain all the physical and electrical properties of the corpuscles. Only the assumption that an atom is a structure that includes not only corpuscular properties, but also quantum ones, could explain the largest number of observed realities.
The corpuscles can be in a bound state, or they can be in a free state. For example, an oxygen atom, to form a molecule, combines with another similar particle. After an electric discharge, such as a thunderstorm, it combines
a more complex structure is azine, which consists of triatomic molecules. Accordingly, certain physical and chemical conditions are necessary for a certain kind of atomic compounds. But there are stronger bonds between the particles of the molecule. For example, a nitrogen atom is connected to another triple bond; as a result, the molecule is extremely strong and almost unchanged.
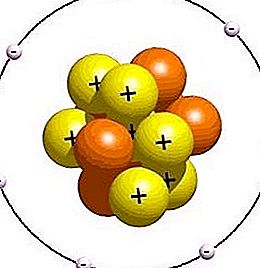
If the number of protons (elementary particles of the nucleus) is similar to the number of electrons rotating in orbits, then the atom is electrically neutral. If there is no identity, then the particle has a negative or positive discharge and is called an ion. As a rule, these charged particles are formed from atoms under the influence of electric fields, radiation of various nature or high temperature. Ions are chemically hyperactive. These charged atoms are able to dynamically react with other particles.




