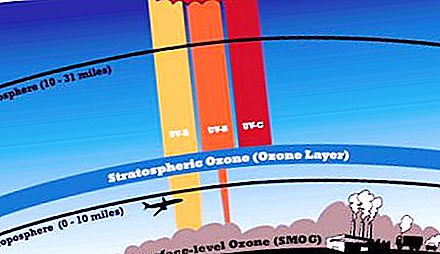
The ozonosphere is a layer of the atmosphere of our planet that delays the most rigid part of the ultraviolet spectrum. Some types of sunlight are detrimental to living organisms. Periodically, the ozonosphere becomes thinner; gaps of various sizes appear in it. Dangerous rays can freely penetrate through the openings on the surface of the Earth. Where is the ozone layer located? What can be done to save it? The proposed article is devoted to the discussion of these problems of geography and ecology of the Earth.
What is ozone?
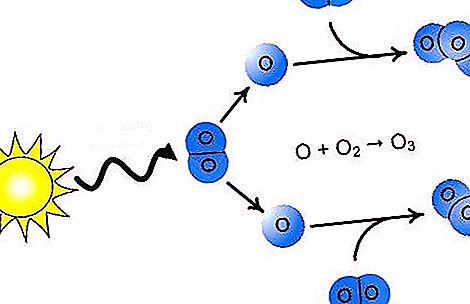
Oxygen on Earth exists in the form of two simple gaseous compounds, is a part of water and a very large number of other common inorganic and organic substances (silicates, carbonates, sulfates, proteins, carbohydrates, fats). One of the more well-known allotropic modifications of the element is the simple substance oxygen, its formula is O 2. The second modification of atoms is O (ozone). The formula of this substance is O 3. Triatomic molecules are formed with an excess of energy, for example, as a result of lightning discharges in nature. Next, we will find out what the ozone layer of the Earth is, why its thickness is constantly changing.
Under normal conditions, ozone is a blue gas with a sharp, specific aroma. The molecular weight of the substance is 48 (for comparison, Mr (air) = 29). The smell of ozone reminds of a thunderstorm, because after this natural phenomenon of O 3 molecules in the air becomes larger. The concentration increases not only where the ozone layer is, but also close to the Earth’s surface. This chemically active substance is toxic to living organisms, but quickly dissociates (decomposes). In the laboratory and industry, special devices have been created - ozonizers - for passing electric discharges through air or oxygen.
What is the ozone layer?
O 3 molecules have high chemical and biological activity. The attachment of the third atom to diatomic oxygen is accompanied by an increase in the energy reserve and instability of the compound. Ozone easily decomposes into molecular oxygen and an active particle, which energetically oxidizes other substances and kills microorganisms. But more often, issues related to the smelling compound relate to its accumulation in the atmosphere above the Earth. What is the ozone layer and why is its destruction harmful?

Directly at the surface of our planet there is always a certain number of O 3 molecules, but the concentration of the compound increases with height. The formation of this substance occurs in the stratosphere due to the ultraviolet radiation of the sun, which carries a large supply of energy.
Ozonosphere
There is an area of space above the Earth where ozone is much larger than near the surface. But in general, the shell consisting of O 3 molecules is thin and discontinuous. Where is the ozone layer of the Earth or the ozonosphere of our planet? The inconsistency in the thickness of this screen has repeatedly confused researchers.
A certain amount of ozone is always present in the Earth’s atmosphere; significant fluctuations in its concentration are observed with height and over the years. We will deal with these problems after we find out the exact location of the protective shield made of O 3 molecules.
Where is the ozone layer of the Earth?
A noticeable increase in the content of ozone molecules begins at a distance of 10 km and remains up to 50 km above the Earth. But the amount of matter that is in the troposphere is not yet a screen. As you move away from the earth's surface, ozone density increases. The maximum values fall on the stratosphere, its area at an altitude of 20 to 25 km. Here, O 3 molecules are contained 10 times more than at the surface of the Earth.
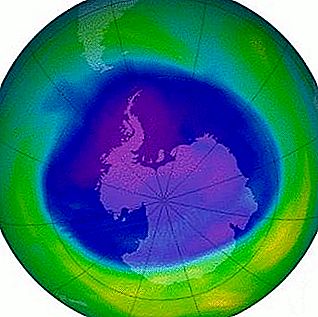
But why is the thickness, integrity of the ozone layer causing concern for scientists and ordinary people? The boom over the state of the protective shield erupted in the past century. Researchers have found that the ozone layer of the atmosphere above Antarctica has become thinner. The main cause of the phenomenon was established - the dissociation of O 3 molecules. The destruction occurs as a result of the combined influence of a number of factors, the leading among them is anthropogenic, associated with the activities of mankind.
Ozone holes
In the last 30–40 years, scientists have noted the appearance of gaps in the protective shield above the surface of the Earth. The scientific community was alarmed by reports that the ozone layer - the Earth’s shield - is actively degrading. All the media in the mid-1980s published reports of a “hole” over Antarctica. Researchers noticed that this gap in the ozone layer increases in the spring. The main reason for the growth of damage was called artificial and synthetic substances - chlorofluorocarbons. The most common groups of these compounds are freons or chladogens. More than 40 substances belonging to this group are known. They come from many sources, because the applications include food, chemical, perfumery and other industries.
The composition of freons, in addition to carbon and hydrogen, includes halogens: fluorine, chlorine, sometimes bromine. A large number of such substances are used as refrigerants in refrigerators, air conditioners. Freons themselves are stable, but at high temperatures and in the presence of active chemical agents enter into oxidation reactions. Among the reaction products may be compounds toxic to living organisms.
Freons and the ozone screen
Chlorofluorocarbons interact with O3 molecules and destroy the protective layer above the Earth's surface. At first, the thinning of the ozonosphere was taken as a natural fluctuation of its thickness, which happens constantly. But over time, holes like a “hole” above Antarctica were seen throughout the Northern Hemisphere. The number of such gaps has increased since the first observation, but they are smaller in size than over the ice continent.
Initially, scientists doubted that it was freons that caused the destruction of ozone. These are substances with a large molecular weight. How can they reach the stratosphere, where the ozone layer is, if it is much heavier than oxygen, nitrogen and carbon dioxide? Observations of ascending flows in the atmosphere during a thunderstorm, as well as experiments conducted, proved the possibility of the penetration of various particles with air to a height of 10–20 km above the Earth, where the boundary of the troposphere and stratosphere is located.
Variety of Ozone Destroyers
Nitrogen oxides resulting from the combustion of fuel in the engines of supersonic aircraft and various types of spacecraft also enter the ozone screen zone. They supplement the list of substances from which the atmosphere, the ozone layer, and the emissions of terrestrial volcanoes are destroyed. Sometimes, gas and dust flows reach a height of 10-15 kilometers and spread hundreds of thousands of kilometers.
Smog over large industrial centers and megacities also contributes to the dissociation of O 3 molecules in the atmosphere. The reason for the increase in the size of ozone holes is also considered to be an increase in the concentration of so-called greenhouse gases in the atmosphere where the ozone layer is located. Thus, the global environmental issue of climate change is directly related to questions about ozone depletion. The fact is that greenhouse gases contain substances that react with O 3 molecules. Ozone dissociates, the oxygen atom causes the oxidation of other elements.
The danger of losing the ozone shield
Were there gaps in the ozonosphere before flying into space, the appearance of freons and other atmospheric pollutants? The listed issues are debatable, but one conclusion is obvious: the ozone layer of the atmosphere must be studied and preserved from destruction. Our planet without a screen of O 3 molecules loses its protection from hard cosmic rays of a certain length absorbed by a layer of active substance. If the ozone screen is thin or absent, then the basic life processes on Earth are in danger. Excessive ultraviolet radiation increases the risk of mutations in the cells of living organisms.
Ozone layer protection
The lack of data on the thickness of the protective shield in past centuries and millennia makes forecasts difficult. What happens if the ozonosphere collapses completely? For several decades, doctors have noted an increase in the number of people affected by skin cancer. This is one of the diseases that causes excessive ultraviolet radiation.
In 1987, several countries acceded to the Montreal Protocol, which provided for a reduction and a complete ban on the production of chlorofluorocarbons. This was only one of the measures that will help preserve the ozone layer - the Earth’s ultraviolet shield. But freons are still produced by industry and enter the atmosphere. However, compliance with the Montreal Protocol has led to a reduction in ozone holes.