Many centuries ago, people guessed that any substance on earth consists of microscopic particles. Some time passed, and scientists proved that these particles really exist. They were called atoms. Usually, atoms cannot exist separately and are combined into groups. These groups are called molecules.

The name "molecule" itself comes from the Latin word moles, meaning heaviness, lump, bulk, and a diminutive suffix - cula. Previously, instead of this term, the word "corpuscle" was used, literally meaning "small body". In order to find out what a molecule is, we turn to explanatory dictionaries. Ushakov’s dictionary says that this is the smallest particle that can exist autonomously and has all the properties of the substance to which it relates. Molecules and atoms surround us everywhere, and although they cannot be felt, all that we see, in fact, is their giant clusters.
Water example
It is best to explain what a molecule is, for example, a glass of water. If half of it is cast, then the taste, color and composition of the remaining water will not change. It would be strange to expect something else. If you cast half again, the amount will decrease, but the properties will again remain the same. Continuing in the same vein, we end up with a small drop. It can still be divided with a pipette, but this process cannot continue indefinitely.
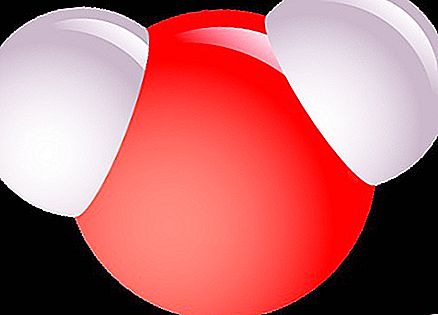
Ultimately, the smallest particle will be obtained, the remainder of the division of which will no longer be water. In order to imagine what a molecule is and how small it is, try to guess how many molecules are in one drop of water. What do you think? Billion? One hundred billion? In fact, there are about a hundred sextillons. This is a number with twenty-three zeros after unity. It is difficult to imagine such a value, therefore, we will use a comparison: the size of one water molecule is smaller than a large apple by as many times as the apple itself is smaller than the globe. Therefore, it cannot be seen even in the most powerful optical microscope.
The structure of molecules and atoms
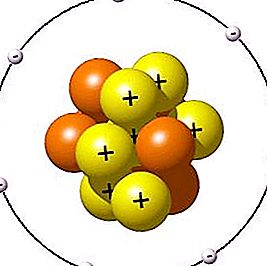
As we already know, all microscopic particles in turn are composed of atoms. Depending on their number, the orbits of the central atoms and the type of bonds, the geometric shape of the molecules can be different. For example, human DNA is twisted in a spiral shape, and the smallest particle of ordinary table salt has the form of a crystal lattice. If a molecule is somehow taken away by several atoms, its destruction will occur. At the same time, the latter will not go anywhere, but will become part of another microparticle.
After we figured out what a molecule is, let's move on to the atom. Its structure is very similar to the planetary system: in the center there is a nucleus with neutrons and positively charged protons, and electrons revolve around in different orbits. In general, an atom is electrically neutral. In other words, the number of electrons is equal to the number of protons.
We hope our article turned out to be useful, and now you no longer have questions about what a molecule and atom are, how they are arranged, and how they differ.




